Table of Contents
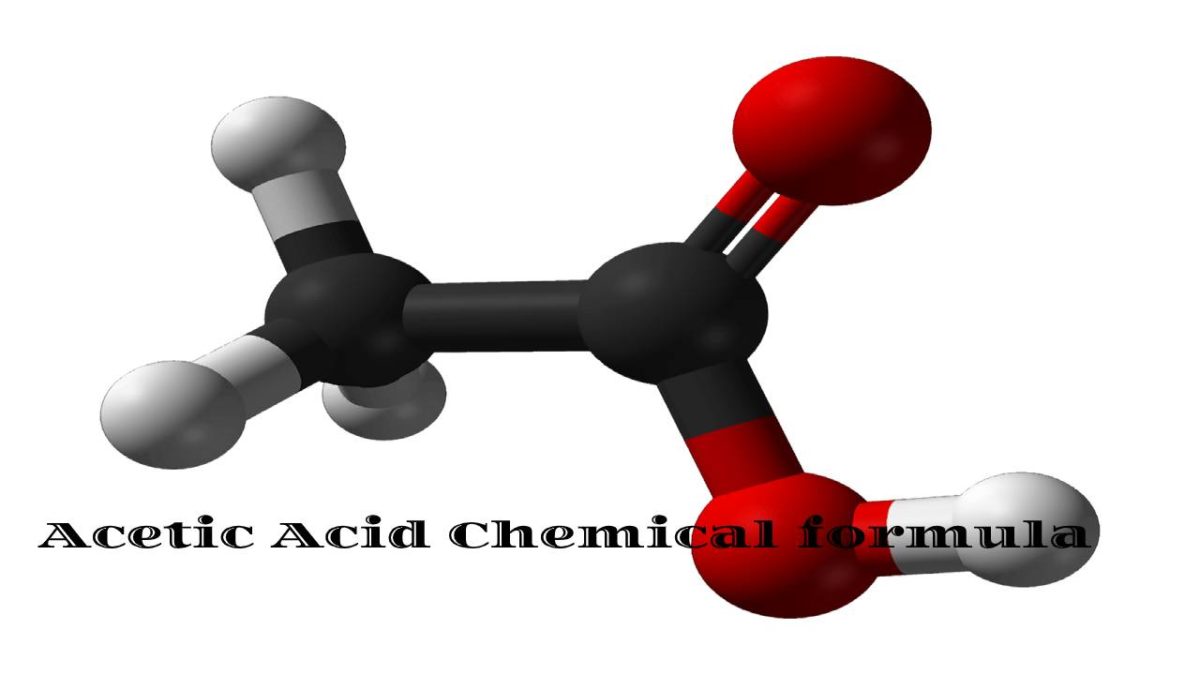
HC2H3O2 is the chemical formula of Acetic acid
HC2H3O2 is the chemical formula of Acetic acid, the organic compound, also called ethanoic acid.
It is a colorless liquid compound that plays an essential role in biological processes.
Its chemical formula is sometimes written as CH3COOH or CH3CO2H to emphasize its atomic organization.
Acetic acid is the least problematic carboxylic acid (next to formic acid) and consists of a single methyl group attached to a carboxyl group.
An acetyl group in a single acetic acid molecule is central to its role in living organisms’ metabolism.
During cell respiration, the acetyl group derived from acetic acid binds to coenzyme A, enabling the metabolism of carbohydrates and fats.
It’s also a natural by-product of products like fruits, grains, rice, and potatoes.
In addition to its crucial role in biology, acetic acid is an essential industrial chemical in manufacturing several consumer products.
Acetic acid is involved in the manufacture of photographic films, adhesives, fibers, fabrics, and cleaning products.
Acetic acid is an additive in the food industry because it has a distinctly sour smell and taste.
After water, it is the second principal component of ordinary household vinegar.
While concentrated acetic acid is safe to consume in diluted quantities, it can damage the skin and internal organs. In addition, you can find the best comparison between Ohaus SPX222 vs. Arlyn SAW-PX on axchangemonitoring.

What is the chemical name of HC2H3O2?
- The chemical name used for HC2H3O2 is Acetic Acid, and most people recognize acetic acid as vinegar when diluted with water.
- HC2H3O2 is an acid, and it is also sometimes used as a solvent.
- It is also used in the mass production of wine, chemicals, starch, and varnish and occurs naturally in plants and animals.
- Because acetic acid can be absorbed when mixed with water, it is not a strong acid, although it can be dangerous in large undiluted amounts.
- Its molecular formula is also C2H4O2 and has a molecular weight of 60.05196.
Chemical properties of acetic acid
- The binding behavior of acetic acid depends on its functional group components.
- Much of acetic acid’s chemical bonding character is based on its carboxyl group and the associated OH group.
- The hydroxyl group enables acetic acid molecules to form hydrogen bonds.
- The hydroxyl group’s polar hydrogen end attracts the polar negative oxygen atom into the carbonyl group of a nearby acetic acid molecule.
- And it creates a strong electrostatic attraction.
- In a solid acetic acid sample, hydrogen bonding’s effect causes molecules to form long, semi-stable chains.
- Acetic acid is an effective solvent and can dissolve not only polar but also non-polar compounds.
- In contrast to water, it is miscible with oils and, like water, can dissolve most organic compounds.
- In terms of chemical reactions, acetic acid reacts in the manner expected for carboxylic acid.
- It forms acetates and water in a primary environment, and it can be reduced by adding hydrogen to create ethanol (alcohol).

- Since acetic acid is acidic, it reacts corrosively with metals to form acetate salts.
- For example, acetic acid reacts with magnesium (Mg) to form magnesium acetate (Mg (CH 3 COOH) 2) and hydrogen gas (H 2).
- Oxidation of metals with acidic compounds produces industrial quantities of hydrogen gas.
Facts about HC2H3O2 is the chemical formula of Acetic acid
- Acetic Acid Facts For Those Who Want To Learn A Little More!
- The Molecular quantity of the Acetic Acid is 60.052 g per mole (g / mol).
- Molecular mass is the total mass of an element or compound (atomic mass), measured in atomic mass units, or “Amu” divided by its amount in moles (moles).
- A single mole is based on the Avogadro number 6.02214076 × 1023.
- This number means that it is easier to compare the mole to the dalton, another scientific unit of atomic mass
Use of Acetic acid
- The uses of acetic acid are diverse. This acid helps in product manufacturing, food processing, cleaning industry, medicine, and hygiene.

- Acetic acid is also an essential biochemical element in the form of an acetyl group. It is fundamental to the construction of amino acids and, therefore, cannot exist without it.
- Let’s take a quick look at sure of these uses for acetic acid.
The HC2H3O2 is the chemical formula of acetic acid in medicine
- If you had an open wound on Kos’s island in the fourth century BC, Hippocrates might have prescribed you a daily vinegar wash.
- Also, if you had a sore throat, you might have queried to mix honey and vinegar to make Oxymel, an ancient Greek cough suppressant.
- If you served in Europe during World War I, you might only have access to vinegar, which will remain clean and infection-free.

Acetic acid as a dietary supplement
- Acetic acid is a popular dietary supplement and is present in vinegar, most commonly apple cider vinegar.
- When it binds to coenzyme A, the acetyl group of acetic acid plays a central role in the metabolism of carbohydrates and fats